Food for Special Medical Purposes (FSMPs)
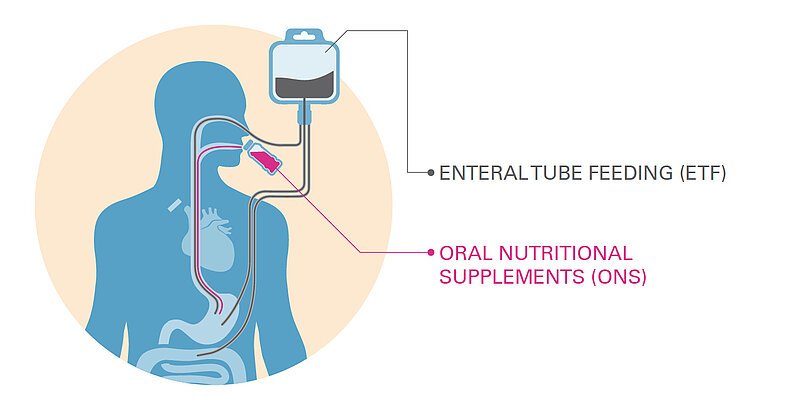
Food for Special Medical Purposes (FSMPs) include Oral Nutritional Supplements (ONS) and Enteral Tube Feeding (EFT).
The definition of FSMPs can vary across regions of the world.
The Codex Standard for the Labelling of and Claims for Foods for Special Medical Purposes (CODEX STAN 180-1991) defines in section 2 (Description) Foods for Special Medical Purposes as a category of foods for special dietary uses which are specially processed or formulated and presented for the dietary management of patients and may be used only under medical supervision. FSMPs are intended for the exclusive or partial feeding of patients with limited or impaired capacity to take, digest, absorb or metabolize ordinary foodstuffs or certain nutrients contained therein, or who have other special medically determined nutrient requirements, whose dietary management cannot be achieved only by modification of the normal diet, by other foods for special dietary uses, or by a combination of the two.
In the EU, FSMPs are one of the food categories governed by EU Regulation No 609/2013 for Foods for Specific Groups (FSG). To supplement the FSG Regulation, specific legislation for FSMPs has been introduced: Commission Delegated Regulation (EU) 2016/128. Within the FSG Regulation, a FSMP is defined as ‘a food specially processed or formulated and intended for the dietary management of patients, including infants, to be used under medical supervision; it is intended for the exclusive or partial feeding of patients with a limited, impaired or disturbed capacity to take, digest, absorb, metabolise or excrete ordinary food or certain nutrients contained therein, or metabolites, or with other medially-determined nutrient requirements, whose dietary management cannot be achieved by modification of the normal diet alone’.
Learn More:
MNI-SNE - 6 easy ways to recognise an FSMP under the EU law (December 2022)
MNI and SNE welcome Commission Notice on the classification of FSMPs (November 2017)
Disclaimer: these documents are intended for general information purposes only and does not constitute legal or other professional advice. It does not replace the relevant EU laws. The information provided is without prejudice to national regulations and interpretations.